
Gwangju Institute of Science and Technology Researchers Design Efficient Iridium Catalyst for Hydrogen Generation
Published by Todd Bush on August 18, 2023
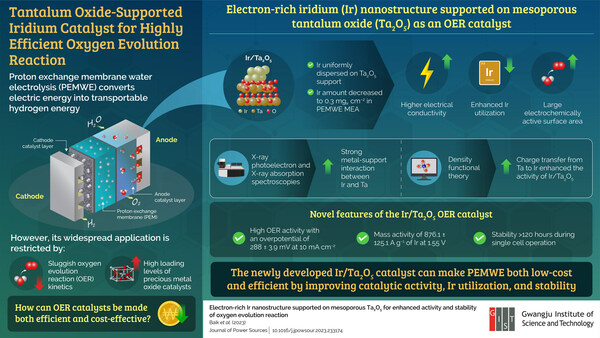
The designed iridium nanostructure, supported on mesoporous tantalum oxide, enhances electrical conductivity, catalytic activity, and long-term stability
GWANGJU, South Korea , Aug. 16, 2023 /PRNewswire/ -- The energy demands of the world are ever increasing. In our quest for clean and eco-friendly energy solutions, transportable hydrogen energy offers considerable promise. In this regard, proton exchange membrane water electrolyzers (PEMWEs) that convert excess electric energy into transportable hydrogen energy through water electrolysis have garnered remarkable interest. However, their widescale deployment for hydrogen production remains limited due to slow rates of oxygen evolution reaction (OER) – an important component of electrolysis – and high loading levels of expensive metal oxide catalysts, such as iridium (Ir) and ruthenium oxides, in electrodes. Therefore, developing cost-effective and high-performance OER catalysts is necessary for the widespread application of PEMWEs.
Researchers from Korea and USA develop a novel iridium catalyst with enhanced oxygen evolution reaction activity, facilitating a cost-effective proton exchange membrane water electrolysis for hydrogen production.
>> In Other News: Fusion Fuel Submits Three Green Hydrogen Projects to Spanish H2 Pioneers II Program
Recently, a team of researchers from Korea and USA, led by Professor Chanho Pak from Gwangju Institute of Science and Technology in Korea, has developed a novel mesoporous tantalum oxide (Ta2O5)-supported iridium nanostructure catalyst via a modified formic acid reduction method that achieves efficient PEM water electrolysis. Their study was made available online on May 20, 2023 and will be published in Volume 575 of the Journal of Power Sources on August 15, 2023. The study was co-authored by Dr. Chaekyung Baik, a post-doctoral researcher at Korea Institute of Science and Technology (KIST).
"The electron-rich Ir nanostructure was uniformly dispersed on the stable mesoporous Ta2O5 support prepared via a soft-template method combined with an ethylenediamine encircling process, which effectively decreased the amount of Ir in a single PEMWE cell to 0.3 mg cm–2," explains Prof. Pak. Importantly, the innovative Ir/Ta2O5 catalyst design not only improved the utilization of Ir but also facilitated higher electrical conductivity and a large electrochemically active surface area.
Additionally, X-ray photoelectron and X-ray absorption spectroscopies revealed strong metal–support interaction between Ir and Ta, while density functional theory calculations indicated a charge transfer from Ta to Ir, which induced the strong binding of adsorbates, such as O and OH, and maintained Ir (III) ratio in the oxidative OER process. This, in turn, led to the enhanced activity of Ir/Ta2O5, with a lower overpotential of 0.385 V compared to a 0.48 V for IrO2.
The team also demonstrated high OER activity of the catalyst experimentally, observing an overpotential of 288 ± 3.9 mV at 10 mA cm−2 and a mass activity of 876.1 ± 125.1 A g−1 of Ir at 1.55 V, significantly higher than the corresponding values for Ir Black. In effect, Ir/Ta2O5 exhibited excellent OER activity and stability, as further confirmed through membrane electrode assembly single cell operation of over 120 hours.
The proposed technology offers the dual benefit of reduced Ir loading levels and an enhanced OER efficiency. "The improved OER efficiency complements the cost-effectiveness of the PEMWE process, enhancing its overall performance. This advancement has the potential to revolutionize the commercialization of PEMWEs, accelerating its adoption as a primary method for hydrogen production," speculates an optimistic Prof. Pak.
Together, this development takes us one step closer to achieving a sustainable transportable hydrogen energy solution and, in turn, carbon neutrality.
Reference
Title of original paper: Electron-rich Ir nanostructure supported on mesoporous Ta2O5 for enhanced activity and stability of oxygen evolution reaction
Journal: Journal of Power Sources
DOI: https://doi.org/10.1016/j.jpowsour.2023.233174
About the Gwangju Institute of Science and Technology (GIST)
http://www.gist.ac.kr/
SOURCE Gwangju Institute of Science and Technology (GIST)
Subscribe to the newsletter
Daily decarbonization data and news delivered to your inbox
Follow the money flow of climate, technology, and energy investments to uncover new opportunities and jobs.
Latest issues
-
One Montana Site Could Supply Half of North America's SAF
Inside This Issue ✈️ Montana's $1.44B Bet on Aviation Fuel Enters Final Stretch 🌍 Carbon Removal Coalition Forms With Goal of Attracting $100-Million in Project Investments 🤝 Prime Minister Carney...
-
Canada Nickel Just Buried CO₂ Before Mining Even Started
Inside This Issue ⛏️ Canada Nickel And UT Prove Mining Can Fight Climate Change 🛰️ OGCI And Carbon Mapper Team Up To Reduce Methane Emissions From The Oil And Gas Sector 🚛 RNG Continues To Lead As...
-
96% Pure H₂ Found Underground — Now What?
Inside This Issue 🧪 HyTerra's Kansas H₂ Could Power a Historic Industry First 🤝 Prime Minister Carney Secures Ambitious New Partnership With India Focused on Energy, Talent, and Technology Françai...
Company Announcements
-
Carbon Removal Coalition Forms With Goal of Attracting $100-million in Project Investments
Leaders in Canada’s nascent carbon-removal industry have joined with several corporate and financial backers as well as the federal government in a bid to attract $100-million in project investment...
-
New Coalition Targets $100M for Canadian Carbon Removal Projects by 2030
An emerging industry to remove carbon dioxide out of the atmosphere got a boost on Thursday with the launch of an initiative to raise another $100 million for those projects. An emerging industry ...
-
TOKYO, March 6, 2026 /CNW/ - Canada is focused on what we can control – strengthening our economy at home and diversifying our partnerships abroad, including in the Indo-Pacific. Japan is an over $...
-
The Government of Canada, BMO, ClimeFi, NorthX, RBC, Shopify, and Vancity launch the "Advance Carbon Removal Coalition" to advance demand for Canadian CDR. OTTAWA, ON, March 5, 2026 /CNW/ - Canada...