
The discovery shows how atomic-scale distortions make fuel-cell catalysts stronger and more efficient for transport.
Researchers in the U.S. have developed a new catalyst that significantly improves the performance and durability of hydrogen fuel cells, potentially making them practical for heavy-duty vehicles and commercial fleets.
The research team at the Brookhaven National Laboratory, a U.S. Department of Energy (DOE) national research institution located in Upton, demonstrated that precise atomic engineering can extend catalyst lifetimes far beyond existing limits.
Tested under stringent conditions, the new material endured more than 90,000 operating cycles, which is the equivalent of 25,000 hours of continuous truck operation, while still outperforming current DOE targets.
>> In Other News: Cold Lake First Nations Raises Concerns Over Carbon Capture Project Potentially Being Put on Federal Fast-track
Xueru Zhao, PhD, a research associate in the lab’s chemistry division, stated that the results point to a practical pathway for developing fuel-cell systems capable of powering the trucks and buses of the future.
“Our catalyst not only meets immediate market needs but also lays the groundwork for widespread adoption in heavy-duty transportation,” Zhao said.
A next-gen catalyst
Fuel cells generate electricity by combining hydrogen and oxygen, releasing only water as a byproduct. This technology already works reliably for passenger cars.
Meanwhile, interest is growing in using them for heavy-duty vehicles like buses, freight trucks and long-haul transport. However, one of the biggest challenges is designing catalysts that are durable and efficient enough to meet the demands of such heavy-duty applications.
“Catalysts are the components that enable the chemistry at the electrodes inside a fuel cell,” Kotaro Sasaki, PhD, a Brookhaven chemist and one of the paper’s lead authors, stated.
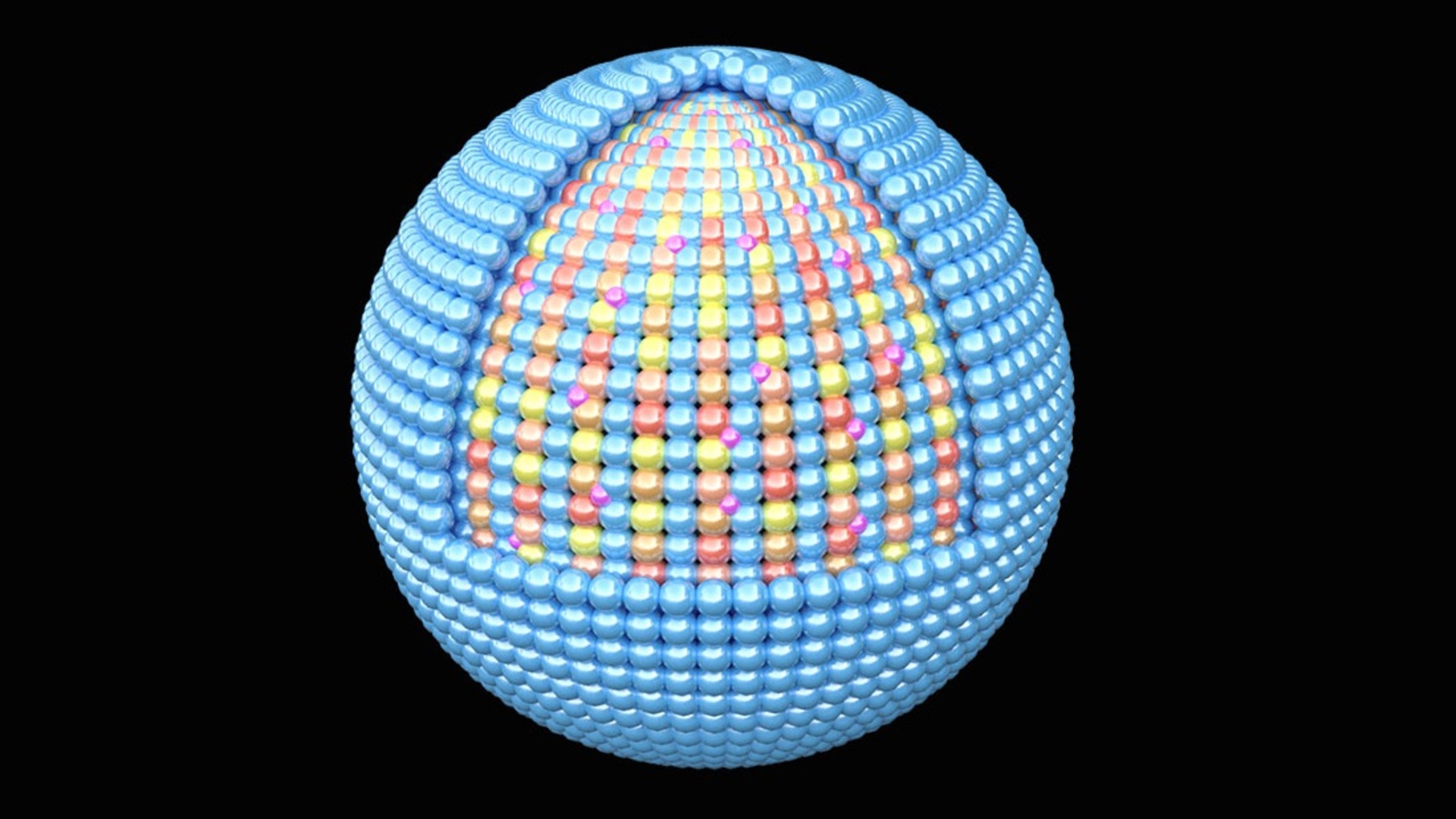
The Brookhaven National Laboratory revealed why a catalyst with a high-entropy intermetallic core, encapsulated by a single-layer shell of Platinum (Pt), shows promise for use in fuel cells for heavy-duty vehicles.
Sasaki revealed that these materials, often made of metals, bring the reacting chemicals together and lower the energy needed to drive the reaction.
“But the catalyst has to be able to perform this function over and over in challenging conditions, such as high heat or a harsh acidic environment,” he explained.
To tackle the challenge, the team came up with a nitrogen-doped catalyst from a finely tuned mix of platinum (Pt), cobalt (Co), nickel (Ni), iron (Fe) and copper (Cu), which is capable of sustaining high performance.
Record performance in tests
The scientists then protected the “high-entropy intermetallic” structure, named for its stable and ordered arrangement of multiple elements, with a platinum monolayer shell.
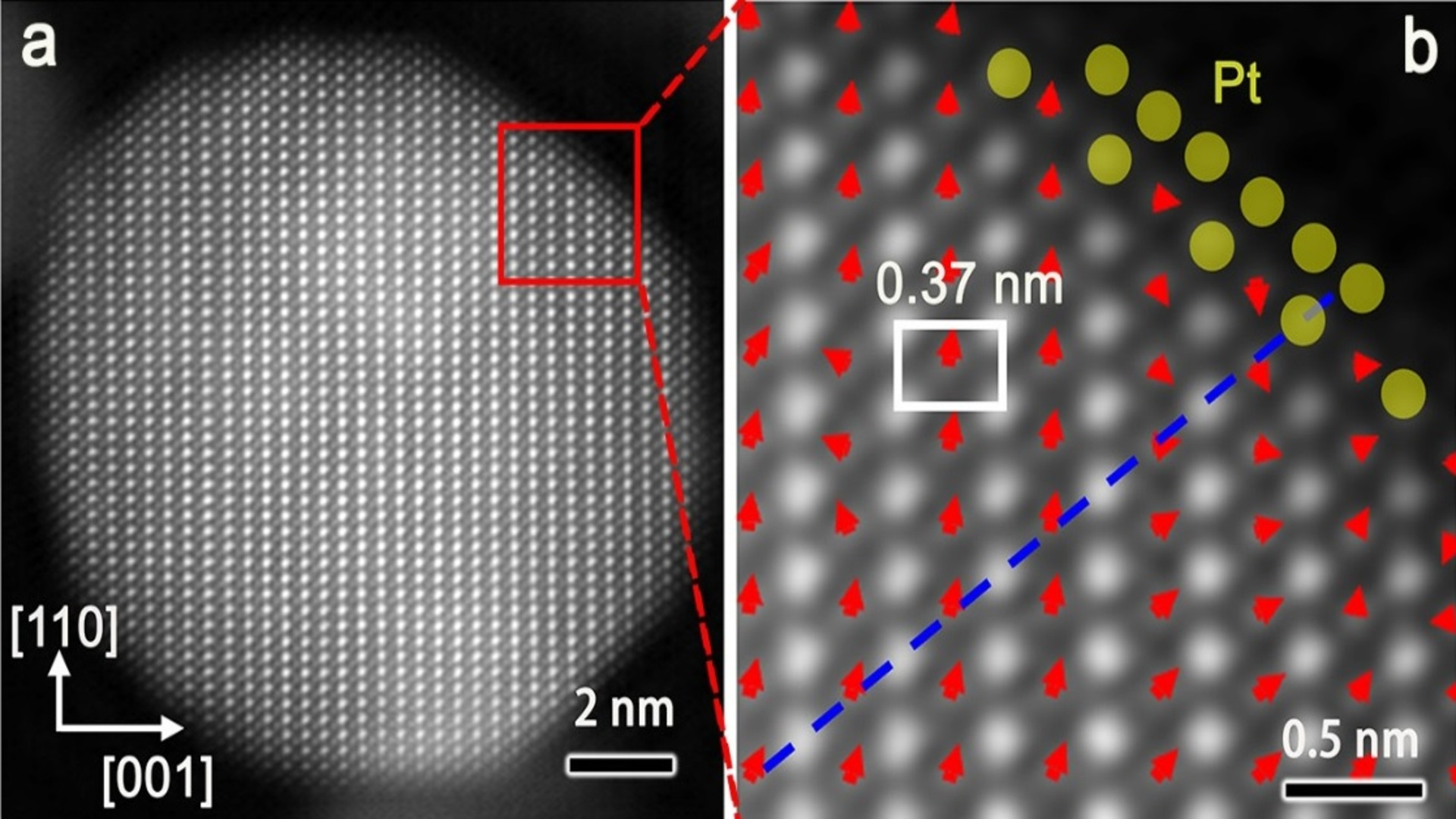
They zoomed in on the material at the atomic level using X-rays and microscopy and discovered that the new catalyst has tiny distortions in its atomic structure, that are partly caused by what they refer to as “sub-angstrom strain.”
These ultra-tiny distortions, which were smaller than the width of a single atom, turned out to be of great importance. They helped create strong bonds between the metals and nitrogen, improving both reactivity and resilience.
Under heavy-duty truck simulation, the new catalyst sustained over 90,000 cycles, equal to 25,000 hours of continuous use, while delivering current densities far above DOE targets.
The achievement involved close cooperation between Brookhaven’s chemistry, physics and nanomaterials divisions, as well as the use of DOE Office of Science user facilities.
“This is a clear example of how fundamental research at a national laboratory can have real-world impact,” Zhao concluded in a press release. “By uncovering the atomic-scale mechanisms that make this catalyst so effective, we’re opening the door to practical technologies that meet industry needs.”
The study has been published in the journal Nature Communications.
Subscribe to the newsletter
Daily decarbonization data and news delivered to your inbox
Follow the money flow of climate, technology, and energy investments to uncover new opportunities and jobs.
Latest issues
-
SAF Output Doubled, So Why Is IATA Sounding Alarms?
Happy New Year from Decarbonfuse! As we wrap up 2025, we want to thank you for being part of the growing Decarbonfuse community. Your engagement and feedback have helped make this platform a trust...
-
$213 Per Tonne: Inside the Latest Multi-Pathway CDR Deal
Inside This Issue 💸 $213 Per Tonne: Inside the Latest Multi-Pathway CDR Deal 🏛️ Clean Energy Technologies Affiliate Vermont Renewable Gas Advances Regulatory Review 💧 Fusion Fuel’s BrightHy Soluti...
-
The Three-Continent Move That Redefines SAF
Wishing everyone a restful holiday season.🎄🎅🎁 Inside this Issue ✈️ Cathay Goes Global With SAF in Three-Continent Fuel Deal 🧪 Proton Ventures Partners With Barents Blue For Realization Of The Bar...
Company Announcements
-
HyOrc Completes Factory Acceptance Test of 500kW ORC Turbine for International Customer
HOUSTON, Dec. 31, 2025 (GLOBE NEWSWIRE) -- HyOrc Corporation (OTCID: HYOR), a clean-energy technology company, today announced the successful completion of the Factory Acceptance Test (FAT) for its...
-
Nova Sustainable Fuels Receives Approval to Produce Sustainable Aviation Fuel in Guysborough County
Nova Sustainable Fuels has received environmental assessment approval for the first phase of a project that will see the company develop a renewable energy park in Goldboro, Guysborough County, whe...
-
Darling Ingredients Announces Sale of Approximately $50 Million in Production Tax Credits
IRVING, Texas -- Darling Ingredients Inc. (NYSE: DAR) today announced the sale of approximately $50 million of production tax credits to a corporate buyer. These credits were generated under the In...
-
Aemetis Receives Funds From the Sale of $17 Million of Federal Clean Energy Tax Credits
CUPERTINO, Calif., Dec. 30, 2025 (GLOBE NEWSWIRE) -- Aemetis, Inc. (NASDAQ: AMTX), a renewable natural gas and renewable liquid fuels company focused on lower cost and reduced emissions products, t...